Study Overview
The BOSTON-1 and BOSTON-2 Phase III clinical research trials will:
- Investigate the safety and the effectiveness of an investigational medicine, Liposomal Cyclosporine A for Inhalation (L-CsA-i), administered via the investigational eFlow® Technology nebulizer system (PARI Pharma GmbH), as a treatment for Bronchiolitis Obliterans Syndrome (BOS) when added to standard of care after lung transplantation
- BOSTON-1 trial is for the treatment of BOS after single lung transplant
- BOSTON-2 trial is for the treatment of BOS after double lung transplant
- Study sites are in US, Austria, Belgium, France, Germany, Israel, Spain, and UK
In each trial, approximately 110 patients will be followed for up to 48 weeks. One group of participants will receive standard of care and the investigational L-CsA-i. The second group will receive their standard of care regimen only and no study medication. Regardless of the treatment allocation, all participants will continue to receive their standard of care regimen for maintenance of the lung allograft. For evaluation, the two groups will be compared.
The primary clinical trial endpoint, or measurement, is the change of lung function over the length of the study. This is measured by a lung function test, called spirometry. One of the key parameters used is the forced expiratory volume in one second (FEV1). FEV1 is a measure of how much air a person can exhale during the first second of the forced breath. Another endpoint is documenting the progression (or worsening) of BOS, or the date of retransplantation, or date of death from respiratory failure.
Medical information and test results are collected throughout the study. This helps with the comparison of those on the study drug versus those in the standard of care only group. If the outcome is favorable for L-CsA-i, the data collected may be submitted to drug regulatory authorities to seek approval of the treatment.
Participants who completed the BOSTON-1 or BOSTON-2 trial will be eligible for screening for the BOSTON-3 open-label extension trial. All individuals enrolled in this extension trial will receive L-CsA-i study medication and standard of care for a period of 2+ years.
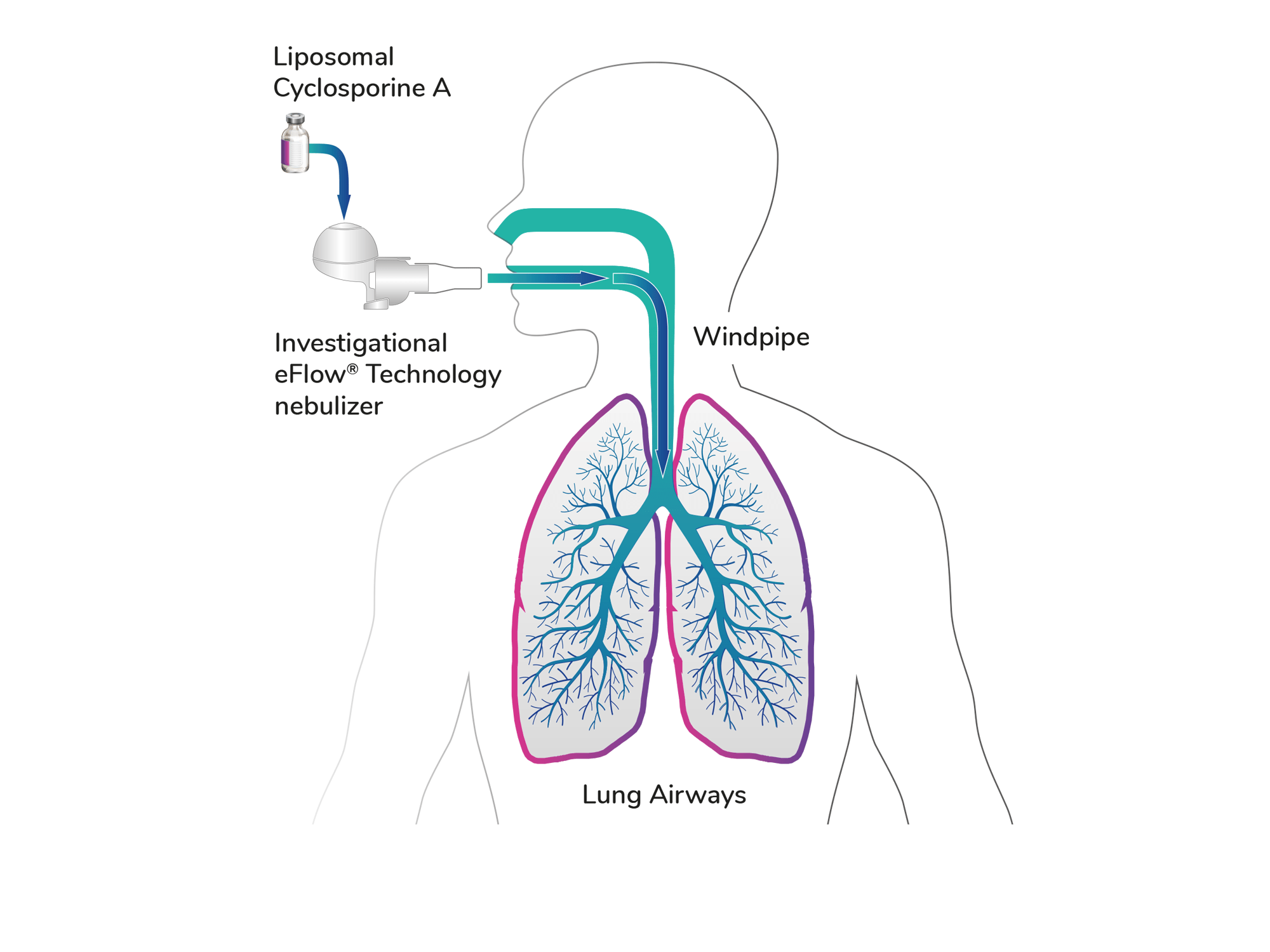
Liposomal Cyclosporine A for Inhalation (L-CsA-i)
- Cyclosporine A is an immunosuppressant drug used to prevent and treat organ rejection after a transplant.
- Liposomal Cyclosporine A for Inhalation (L-CsA-i) is an investigational formulation of cyclosporine A that is enclosed within tiny droplets called liposomes. Liposomes are known drug delivery systems that, when inhaled, may help carry difficult to dissolve medication into the lungs.
- L-CsA-i is administered into the lungs using an optimized nebulizer system.
The BOSTON research studies are being performed to evaluate the safety and efficacy of investigational L-CsA-i as a treatment for Bronchiolitis Obliterans Syndrome (BOS) when added to a standard of care regimen after lung transplantation.
-
Individuals who are interested and consent to be in the trial will be screened to determine if they are eligible to participate.
-
If they qualify, they will be randomized (like flipping a coin) and assigned to one of two groups. There is an equal (50/50) chance of being placed into either study group.
-
A computerized system determines which study group an individual will be placed.
-
One group will receive standard of care and the investigational L-CsA-i. The second group will receive standard of care and no study medication.
-
Participants will know which group they have been assigned to after they qualify and enroll in the study. Most of the study staff will also know the participant’s treatment assignment.
-
There is no cost to be in the trial and participants will be reimbursed for their expenses for travel to and from the study site.
For someone to take part in the BOSTON trial, the study doctor will need to review the full checklist of criteria specified in the study protocol. Some basic conditions that must be met include:
- Are over the age of 18 years
- Have had a single or double lung transplant
- Have been diagnosed with bronchiolitis obliterans syndrome and there are signs of worsening of the condition
- Are receiving a standard immunosuppression regimen
- Are willing to commit to visits with the study doctor
- Meet all other requirements to participate in the trial